 08045816368
08045816368
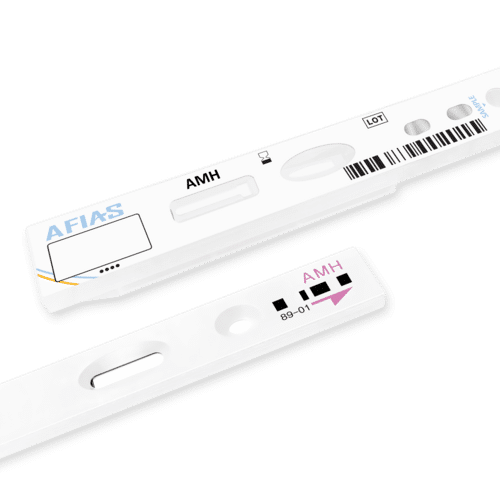
Boditech ichroma AMH test kit
650 INR/Kit
Product Details:
- Accuracy >95%
- Features Rapid, accurate, reproducible, easy to use, minimal sample volume
- Display Type Analyzer-based digital display
- Measurement Range 0.08 - 20.00 ng/mL
- Instruments Type Fluorescent immunoassay test kit
- Frequency Single use test strips
- Storage Instructions Store at 2-8C, avoid freezing
- Click to view more
X
Boditech ichroma AMH test kit Price And Quantity
- 650 INR/Kit
- 2 Box
- Within 12 minutes
- 25 test cartridges, ID chips, sample diluents, instruction manual
- Professional laboratory personnel
- Barcode on kit and cartridges
- ichroma II, ichroma 50, other Boditech fluorescence analyzers
- 75 L
- 20% to 80% RH
Boditech ichroma AMH test kit Product Specifications
- 0.08 - 20.00 ng/mL
- Fluorescence Immunoassay (FIA)
- Silent Operation
- Kit box: approx. 200 x 150 x 100 mm
- Plastic, reagents, buffer solutions
- Fluorescent immunoassay test kit
- Single use test strips
- Rapid, accurate, reproducible, easy to use, minimal sample volume
- >95%
- Analyzer-based digital display
- White and orange labeling
- No
- Laboratory & Point of Care
- Uses compatible ichroma fluorescence analyzer
- 18 months
- Automated/Manual
- Depends on analyzer (100-240V, 50/60Hz)
- Depends on analyzer
- New
- Clinical Diagnostic Kit
- Yes
- Quantitative AMH (Anti-Mllerian Hormone) detection in human serum or plasma
- Approx. 0.5 kg per kit
- Store at 2-8C, avoid freezing
- Yes
- Detection and quantification of AMH
- Within 12 minutes
- 25 test cartridges, ID chips, sample diluents, instruction manual
- Professional laboratory personnel
- Barcode on kit and cartridges
- ichroma II, ichroma 50, other Boditech fluorescence analyzers
- 75 L
- 20% to 80% RH
Boditech ichroma AMH test kit Trade Information
- Cash Advance (CA), Cash in Advance (CID), Cheque, Telegraphic Transfer (T/T)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India
Product Description
Evaluating ovarian function & predicting controlled ovarian hyperstimulationAMH levels help predict the remaining egg reserve. This can tell a lot about the reproductive issues, including the onset of menopause, amenorrhea, polycystic ovary syndrome. This test also tells the expected response to controlled ovarian hyperstimulation to achieve pregnancy in IVF.
Fast & Reliable AMH Quantification
The ichroma AMH test kit offers rapid results in under 12 minutes with precision exceeding 95%. Its fluorescence immunoassay technology ensures high accuracy, supporting timely and informed clinical decisions. The kit requires a minimal sample volume, making it particularly beneficial for laboratories dealing with limited specimens or pediatric samples.
User-Friendly Operation & Versatile Application
Designed for both professional laboratory personnel and point-of-care testing, this kit supports automated and manual operation. Clear instructions and included accessories simplify the testing process. Its compact, portable design and compatibility with multiple Boditech analyzers make it a flexible solution for various clinical environments.
Consistent Quality & Optimal Storage
Every kit features unique barcodes on cartridges and packaging for reliable lot traceability. To maintain reagent integrity, store the kit at 2-8C and avoid freezing. The shelf life extends to 18 months, allowing laboratories to manage inventory with confidence and ensure consistent, reproducible outcomes.
FAQ's of Boditech ichroma AMH test kit:
Q: How is the Boditech ichroma AMH test kit utilized in a laboratory setting?
A: The kit is designed for quantitative detection of AMH in human serum or plasma. Simply add 75 L of sample to the cartridge, mix with the provided diluent, and insert into a compatible ichroma fluorescence analyzer. The process is streamlined for both automated and manual workflows.Q: What are the main benefits of using this AMH test kit?
A: Key benefits include rapid turnaround (results within 12 minutes), high accuracy (>95%), minimal sample requirement (75 L), and compatibility with various Boditech analyzers. It delivers reliable quantification for clinical decision-making in fertility assessment and related diagnostics.Q: When should I use the ichroma AMH test?
A: Use this test when quantifying Anti-Mullerian Hormone levels is required, such as in fertility evaluations, ovarian reserve determination, or monitoring certain medical conditions. It is suitable for both laboratory and point-of-care use.Q: Where can the ichroma AMH kit be implemented?
A: This kit is intended for use by professional laboratory personnel in clinical diagnostic labs, fertility centers, and healthcare facilities where Boditech fluorescence analyzers are available. Its portable nature also facilitates point-of-care testing.Q: What is the recommended storage condition for the kit?
A: Store the kit at 2-8C and avoid freezing. Ensure the storage area maintains a relative humidity between 20% and 80% to preserve the integrity and shelf life of the reagents and test components.Q: How does the test achieve reliable traceability?
A: Each kit and its cartridges are equipped with unique barcodes, allowing for thorough lot tracking and traceability through the laboratory's quality management systems, ensuring compliance and reliable records.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email






 Send Inquiry
Send Inquiry Send SMS
Send SMS
