 08045816368
08045816368
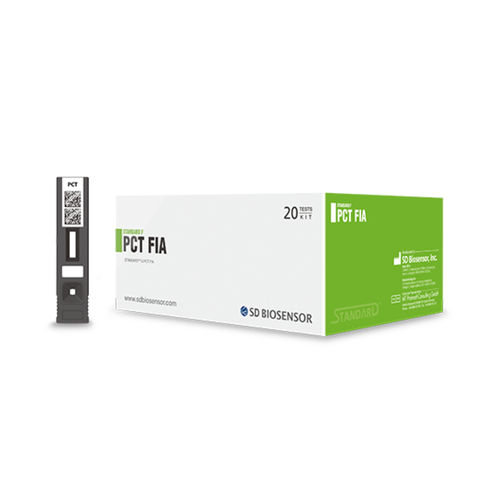
STANDARD F PCT FIA test kit
330 INR/Kit
Product Details:
- Accuracy 95%
- Display Type Result read by STANDARD F FIA Analyzer Display
- Instruments Type Rapid Test Kit for FIA Platform
- Usage Type Professional, Laboratory, Point of Care
- Shelf Life 18 months from manufacturing date
- Function Detection and quantification of PCT in human serum/plasma/whole blood samples
- Measurement Range 0.1100 ng/mL
- Click to view more
X
STANDARD F PCT FIA test kit Price And Quantity
- 330 INR/Kit
- 1 Box
- Dispose biohazard as per local regulations
- STANDARD F Analyzer (SD BIOSENSOR)
- 0.1 ng/mL
- Serum, Plasma, Whole Blood
- Indicated on box
- 25 Test Devices, 25 Sample Tubes, 25 Dropper Tips, Buffer Solution, Package Insert, ID Chip
- Sandwich immunodetection based on fluorescent technology
- Use test cassette immediately after opening (within 1 hour)
- Printed on kit packaging
- For In-Vitro Diagnostic use only
- Within 15 minutes
- 75 L
- Instrument-based (Analyzer Required)
STANDARD F PCT FIA test kit Product Specifications
- FIA Test Kit
- New
- Approx. 270 g per kit box
- 0.1100 ng/mL
- Rapid test, Easy handling, High sensitivity and accuracy, For Professional Use Only
- Store at 230C, Keep away from direct sunlight, Do not freeze
- White cassette, Printed packaging
- 95%
- Quantitative detection of Procalcitonin (PCT)
- Plastic (Cassette housing), Medical Grade Components
- Yes
- Fluorescent Immunoassay (FIA)
- Kit Box: Approx. 220 mm x 110 mm x 70 mm
- Result read by STANDARD F FIA Analyzer Display
- Yes
- Silent Operation
- Not Applicable to Kit
- Rapid Test Kit for FIA Platform
- Professional, Laboratory, Point of Care
- Manual Preparation, Automatic Reading by Compatible FIA Analyzer
- Detection and quantification of PCT in human serum/plasma/whole blood samples
- 18 months from manufacturing date
- Not Applicable (Requires FIA Analyzer for testing)
- No
- Dispose biohazard as per local regulations
- STANDARD F Analyzer (SD BIOSENSOR)
- 0.1 ng/mL
- Serum, Plasma, Whole Blood
- Indicated on box
- 25 Test Devices, 25 Sample Tubes, 25 Dropper Tips, Buffer Solution, Package Insert, ID Chip
- Sandwich immunodetection based on fluorescent technology
- Use test cassette immediately after opening (within 1 hour)
- Printed on kit packaging
- For In-Vitro Diagnostic use only
- Within 15 minutes
- 75 L
- Instrument-based (Analyzer Required)
STANDARD F PCT FIA test kit Trade Information
- JAIPUR
- Telegraphic Transfer (T/T), Cash Advance (CA), Cash in Advance (CID)
- 10 Days
- Contact us for information regarding our sample policy
- Africa, Asia, Australia, Central America, Eastern Europe, Middle East, North America, South America, Western Europe
- All India
Product Description
STANDARD F PCT FIA is a fluorescent immunoassay for the quantitative measurement of procalcitonin level in human serum, plasma, and whole blood. Procalcitonin helps assess the severity and prognosis of bacterial infections, and support early diagnosis of sepsis.Advantage
- Fluorescent technology- Optimized performance
- Reagents can be stored at room temperature
- No-coding via 2D barcode system
Advanced Fluorescent Technology
The STANDARD F PCT FIA test kit employs sandwich immunodetection based on fluorescent technology, ensuring rapid and highly sensitive results. This innovative approach enables reliable quantification of Procalcitonin levels, supporting clinicians in making informed decisions efficiently within minutes.
Easy Operation, Precise Results
Designed for professional use, the kit requires only 75 L of sample and minimal manual preparation. The result is automatically read by the compatible STANDARD F Analyzer, eliminating subjectivity and providing accurate measurements every time. The process is streamlined for both laboratory and point-of-care applications.
Comprehensive Kit for Professional Settings
This product includes 25 test devices, sample tubes, dropper tips, buffer solution, an ID chip, and a package insert, making it ready to use out of the box. All components meet medical grade standards, and detailed instructions ensure simple yet effective operation in various clinical environments.
FAQ's of STANDARD F PCT FIA test kit:
Q: How does the STANDARD F PCT FIA test kit work?
A: The test kit uses sandwich immunodetection with fluorescent technology to detect and quantify Procalcitonin (PCT) in serum, plasma, or whole blood samples. After adding the sample and reagents, the STANDARD F Analyzer reads the fluorescence signal and calculates PCT concentration within 15 minutes.Q: What is the intended use of this PCT test kit?
A: This kit is intended for in-vitro quantitative detection of Procalcitonin (PCT) as a rapid aid in diagnosing bacterial infections and sepsis, primarily for use by laboratory professionals and healthcare practitioners in professional or point-of-care settings.Q: What sample types and volume are required for testing?
A: The kit is compatible with serum, plasma, and whole blood samples. For each test, a sample volume of 75 L is required.Q: How soon are results available and how are they interpreted?
A: Results are available within 15 minutes and are interpreted instrumentally using the STANDARD F Analyzer. The analyzer automatically displays quantitative results on its screen, eliminating manual interpretation.Q: What are the storage requirements for the kit and opened cassettes?
A: The kit should be stored at 2-30C, kept away from direct sunlight, and should not be frozen. Test cassettes must be used immediately after opening, ideally within one hour, to ensure result accuracy.Q: Where can the STANDARD F PCT FIA test kit be used?
A: This kit is suitable for professional environments including laboratories and point-of-care settings. It is not intended for home or self-use, as operation and interpretation require compatible instrumentation and professional expertise.Q: What are the main benefits of using this test kit?
A: Benefits include rapid turnaround time (results within 15 minutes), high sensitivity and accuracy (95%), minimal sample required, and simple handling. The automated analyzer reading ensures precise and objective results for better patient management.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email





 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free
